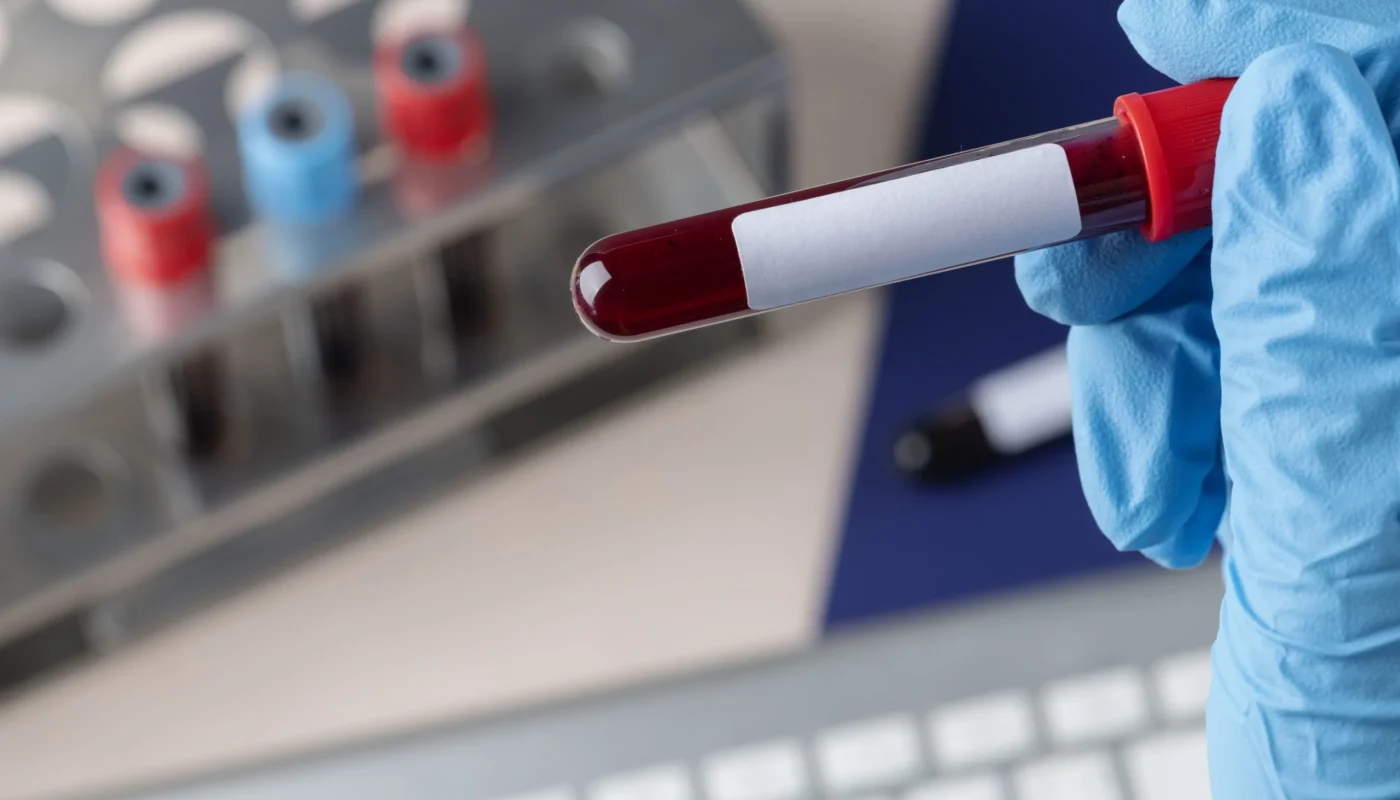
A breakthrough study published in Cancer Discovery has demonstrated the effectiveness of a new blood test in detecting cancer at an early stage in patients with Li-Fraumeni Syndrome. This multimodal liquid biopsy assay, which utilizes cell-free DNA (cfDNA), outperformed traditional unimodal assays and also detected cancer-related signals prior to conventional diagnosis.
Li-Fraumeni Syndrome (LFS) is a hereditary condition caused by mutations in the TP53 gene, which increases the risk of developing multiple types of cancer by up to 100%. Early detection is crucial for improving outcomes in cancer patients, but intensive surveillance in LFS patients is hindered by physical, economic, mental, and logistical barriers.
Liquid biopsy has emerged as a promising solution that offers a less invasive method for detecting cancer-related genetic signatures, such as circulating tumor DNA (ctDNA) in blood samples. This eliminates many of the logistical barriers associated with testing.
To address the lack of data on the use of cfDNA in high-risk groups like LFS patients, researchers developed a cfDNA-based multimodal approach to detect a range of cancer-related signals.
The study involved collecting 193 venous blood samples (154 cancer-negative and 39 cancer-positive) from 89 individuals with a TP53 mutation. The patients were divided into different categories based on their cancer history. Various DNA analysis techniques were used to determine the sensitivities and specificities of the individual and integrated assays.
The results showed that TP53 mutation carriers had a higher proportion of short DNA fragments, with an even greater increase seen in cancer-positive individuals. Integrating genomic, fragmentomic, and epigenomic analysis allowed for the detection of cancer-related signals in 81.6% of samples, resulting in a significant improvement in sensitivity compared to unimodal analysis.
The study also showed that the cfDNA approach had a negative predictive value of 95.4% in cancer-free TP53 mutation carriers, indicating its ability to accurately identify those without cancer. However, further follow-up is needed to confirm the actual false positive rate among these patients.
When analyzing individual LFS cases, the multimodal approach was found to be as sensitive or even more effective than conventional clinical screening methods. This suggests that the incorporation of cfDNA-based diagnosis could complement existing diagnostic and management strategies for cancer, especially in high-risk populations.
One of the major advantages of the integrated cfDNA tests is their less invasive nature, which allows for increased testing frequency among high-risk patients. However, the study was limited by its retrospective design and the low adoption rate of cfDNA analysis in clinical settings compared to other methods.
Further research is needed to clinically validate the use of cfDNA in LFS patients and confirm its impact on improving outcomes.
The findings of this study offer significant hope for individuals with Li-Fraumeni Syndrome, providing a non-invasive and effective method for early cancer detection. By incorporating cfDNA tests alongside traditional screening modalities, the sensitivity and accuracy of cancer detection can be optimized, leading to better care for high-risk patients.
Note:
- Source: Coherent Market Insights, Public sources, Desk research
- We have leveraged AI tools to mine information and compile it