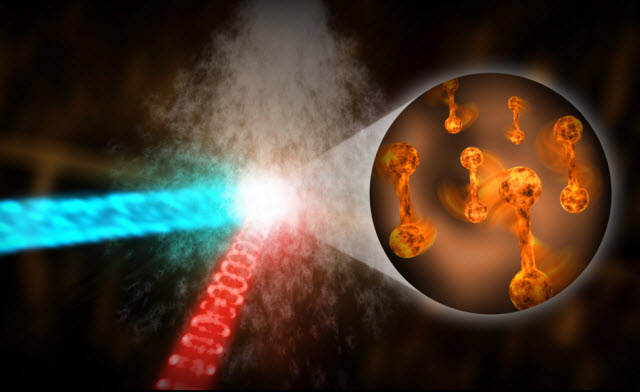
Scientists from the Department of Energy’s SLAC National Accelerator Laboratory and Stanford University have successfully captured hydrogen atoms in motion using ultrafast electron diffraction (UED). The results of their study, published in Physical Review Letters, provide valuable insights into proton transfers, which are crucial in numerous biological and chemical reactions.
Proton transfers play a vital role in various reactions in biology and chemistry, including enzymatic reactions and energy production in mitochondria. Understanding the structure of protons during these reactions is essential, but the fast-paced nature of proton transfers makes them challenging to observe. These transfers occur within femtoseconds, which are one millionth of one billionth of a second.
To overcome this challenge, the researchers turned to UED as a powerful tool. They utilized high-energy Megaelectronvolt (MeV) electrons to study hydrogen atoms and proton transfers. In their experiment, they used ammonia molecules, which consist of a nitrogen atom bonded to three hydrogen atoms. The team subjected the ammonia molecules to ultraviolet light, causing one of the hydrogen-nitrogen bonds to break. They then directed a beam of electrons through the dissociated ammonia and captured the diffracted electrons.
Notably, the researchers were able to observe the separation of hydrogen from the nitrogen nucleus and track changes in the molecule’s structure. By analyzing the scattered electrons at different angles, they could distinguish between the signals related to the electrons and the atomic nuclei.
This combination of sensitivity to electrons and atomic nuclei in a single experiment is highly valuable. Understanding the sequence of events during atom dissociation, whether the electrons or the nuclei initiate the separation, can provide insights into the mechanism of dissociation reactions.
The findings could lead to a better understanding of proton transfers and their implications in chemistry and biology. The ability to observe the behavior of protons could be particularly beneficial in structural biology, where traditional techniques like X-ray crystallography and cryo-electron microscopy struggle to capture proton movements.
In future studies, the research team plans to replicate the experiment using X-rays at SLAC’s X-ray laser, the Linac Coherent Light Source (LCLS), to compare the results. Additionally, they aim to enhance the intensity of the electron beam and improve the time resolution to observe individual steps of proton dissociation over time. These advancements could further unravel the mysteries of proton transfers and their role in fundamental biological and chemical processes.