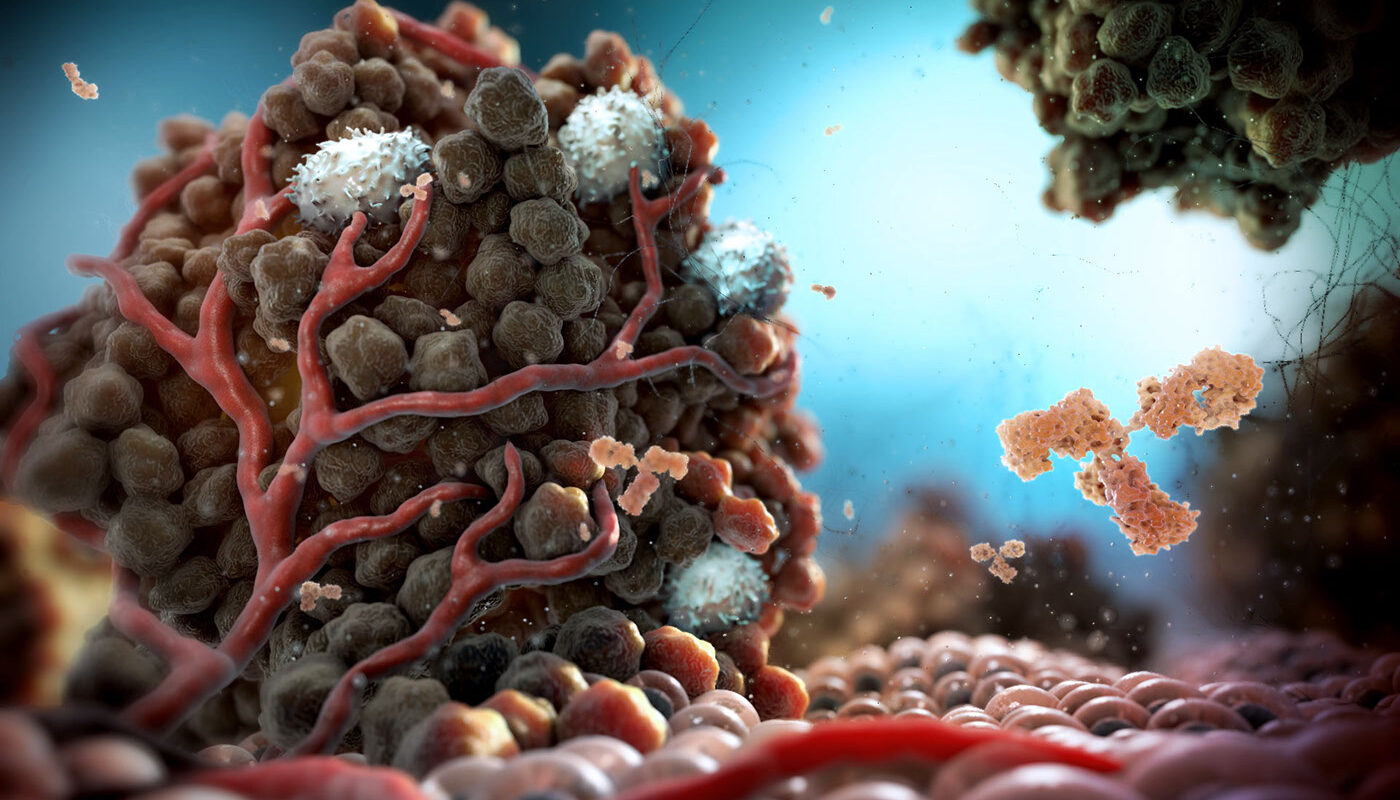
The microenvironment surrounding a tumor plays a critical role in cancer progression. Often referred to as the tumor microenvironment, it consists of various cell types that interact both with each other and with cancer cells. This interplay between the tumor and its microenvironment ultimately shapes how the disease develops and spreads.
Components of the Tumor Microenvironment
The tumor microenvironment contains a variety of cells in addition to cancer cells. Immune cells that attempt to fight the tumor are present, such as macrophages, lymphocytes, and myeloid-derived suppressor cells. Other cell types include cancer-associated fibroblasts, pericytes that surround blood vessels, endothelial cells that line blood vessels, and mesenchymal stem cells. The extracellular matrix where these cells reside provides physical structure and molecular signals. Growth factors, cytokines, and enzymes that remodel the extracellular matrix are part of the chemicals exchanged. Together, these cellular and extracellular components form a dynamic network surrounding the tumor.
Immune Cells are Actively Recruited and Manipulated
Immune cells play a crucial yet complex role in cancer progression. Tumor Microenvironment find ways to subvert anti-tumor immunity and instead promote tumor growth. Macrophages are recruited by the tumor which then “reprograms” them into tumor-associated macrophages that aid the cancer. Myeloid-derived suppressor cells are expanded in cancer and powerfully suppress T cell responses against the tumor. Chemokines and other factors secreted by tumors attract these cell types and skew them towards immune suppression. Cancer cells have devised molecular and cellular strategies to evade detection and killing by immune effector cells that would normally destroy forming tumors.
Cancer-Associated Fibroblasts Alter the Tumor Environment
Fibroblasts are cells that maintain structural and biochemical support within tissues. In cancer, some fibroblasts acquire features associated with wound healing and tissue remodeling. Called cancer-associated fibroblasts, they influence the tumor microenvironment in several key ways. First, they deposit components of the extracellular matrix including collagen, fibronectin, and hyaluronan which remodel the architecture surrounding cancer cells. This facilitates tumor growth, invasion, and migration. Cancer-associated fibroblasts also secrete growth factors, chemokines, and enzymes that fuel these processes. For example, factors like TGF-β, PDGF, and FGF released by fibroblasts stimulate cancer cell proliferation and migration. Together these properties of cancer-associated fibroblasts profoundly influence tumor progression and invasiveness.
Blood Vessel Formation is Induced
Tumors Microenvironment co-opt the body’s normal responses to induce blood vessel growth, a process known as angiogenesis, to support their metabolic needs as they expand in size. Cancer cells and fibroblasts secrete multiple pro-angiogenic factors such as VEGF, FGF, PDGF, and angiopoietins which influence endothelial cells. In response to these signals, nearby resident endothelial cells initiate angiogenesis. Sprouting involves endothelial cell proliferation, migration, invasion of surrounding tissues, and formation of tubular networks. Pericytes are also recruited to wrap around and stabilize these nascent vessels. Without this induced blood vessel formation, beyond a certain size solid tumors would outgrow their local blood supply and nutrient diffusion limits, resulting in necrosis.
Metastatic Niches are Prepared at Distant Sites
Metastasis, the spread of cancer from the primary site to other organs, requires assistance from the secondary site’s microenvironment. Factors released from the primary tumor educate distant tissues and attract cancer cells. Once metastasis seeds arrive, they encounter tissue environments preconditioned by these factors and are supported for survival and growth. For instance, CXCL12 released from primary breast cancers prepares lungs for their arrival by activating expression of CXCL12 in lung tissue. This recruits cancer cells to the lungs via CXCR4. Additionally, factors derived from primary tissues mobilize bone marrow-derived cells that travel to and reconstitute pre-metastatic niches to aid metastatic colonization. Thus, primary tumors actively shape microenvironments at secondary sites to enable metastatic outgrowth.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it