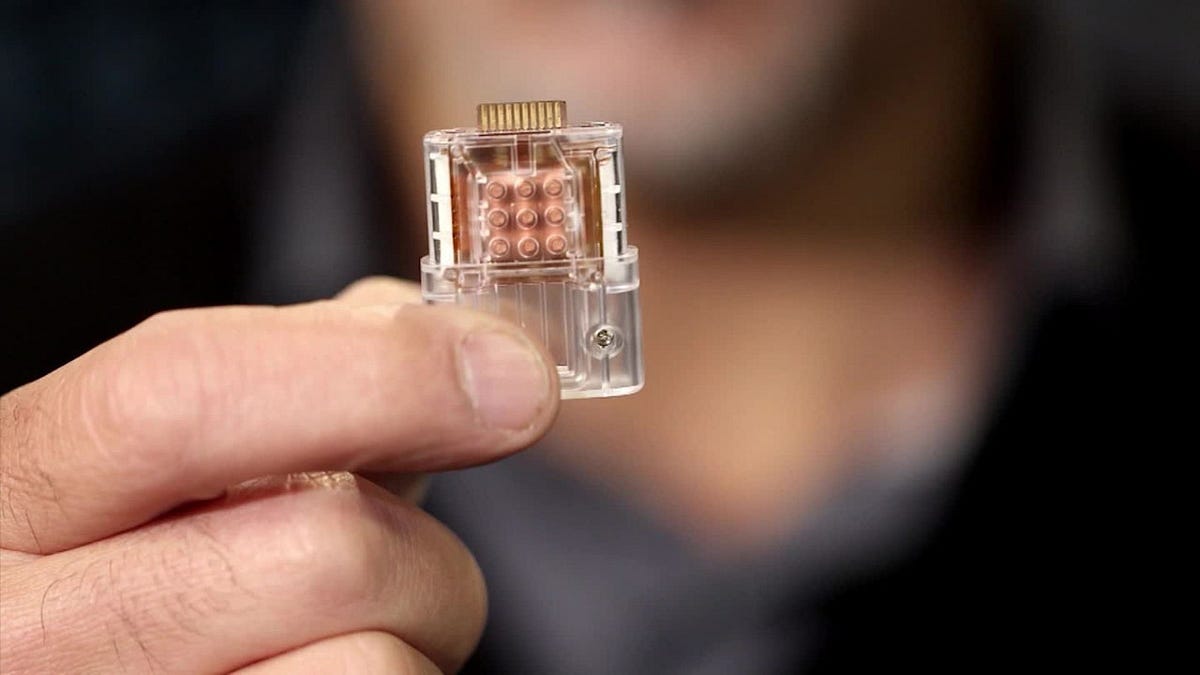
The biomedical industry has witnessed significant growth in the use of biochip products and services owing to advancements in microfluidics technologies. Biochips are compact devices that integrate numerous biochemical reactions into a single integrated circuit to perform complex analyses such as disease diagnosis, genetic testing, drug discovery, and other biomedical applications. The miniaturized nature of biochips enables high-throughput analysis for a multitude of samples simultaneously on a microfluidic chip, thus reducing reagent costs, processing time, and labor. Biochips offer advantages such as improved portability, reduced sample size, low power consumption, and enhanced functionality. The growing demand for point-of-care disease diagnosis and personalized medicine is propelling the need for biochip technologies.
The U.S. biochip product and services market is estimated to be valued at US$ 8.76 Mn in 2024 and is expected to exhibit a CAGR of 6.3% over the forecast period 2024 to 2031.
Key Takeaways
Key players operating in the US biochip product and services are Galderma SA, Allergan plc (AbbVie Inc.), Bayer AG, Pfizer Inc., Leo Pharma A/S, Sol-Gel Technologies Ltd., Foamix Pharmaceuticals Ltd., Mayne Pharma, Group Limited, AnaptysBio, Inc., Mylan N.V.
Key opportunities in the market include increased focus on developing countries, rising demand for biochips in drug discovery and development, and growing technological advancements in biochip design and fabrication. Technological advancements such as improved microarray processing, nanotechnology integration, lab-on-a-chip development, and molecular diagnostics are expanding the applications of biochips.
Market drivers
The primary drivers contributing to the growth of the US Biochip Product And Services Market Size are increasing incidence of chronic and infectious diseases worldwide, rising demand for point-of-care diagnosis, and growing government support for biochip R&D programs. Healthcare providers are increasingly adopting biochip technologies for early-stage disease diagnosis, expedited treatment, and preventive care. Burgeoning applications in medical diagnostics, drug discovery, and genomic research are also fueling market growth. Rising healthcare expenditure, growing demand for personalized medicine, and increasing adoption of microfluidics techniques in the biomedical sector are some other factors accelerating the demand for biochip products and services.
Current challenges in the US biochip product and services market:
The US biochip product and services market is facing various challenges which are hampering its growth. One of the major challenges is the high cost associated with biochip production and services. Developing advanced biochips require extensive research and development activities which increases the overall costs. As a result, biochip-based services and solutions become expensive for end-users. Another challenge is the lack of standardized regulations. The regulatory framework varies across different states in the US which creates confusion among market players. Stringent approval procedures further increase the time required to commercialize new biochips. Lack of skilled professionals is also a critical challenge as developing and analyzing data from biochips require specialized training. This skills gap makes it difficult for companies to scale up their operations.
SWOT Analysis
Strength: The US has a well-developed healthcare infrastructure and high healthcare spending which provides potential for market growth. Significant funding from government entities and private investors makes the country an attractive market for R&D activities.
Weakness: High dependency on imports from other countries like Germany and UK for certain biochip components. Established market players face intense competition from emerging low-cost manufacturers.
Opportunity: Growing demand for personalized medicine is driving the need for Point-of-Care testing and diagnosis using biochip-based devices. Increasing adoption of biochips in drug discovery and development activities by pharmaceutical companies provides new opportunities.
Threats: Stringent regulations can delay product approvals and market entry. High production costs remain a challenge especially for diagnostic biochips targeting price-sensitive markets. Intense competition from alternative technologies threatens the growth of certain biochip segments.
The US west coast region consisting of states like California, Washington and Oregon accounts for over 40% of the total biochip product and services market value in the US. This is due to the high concentration of technology companies and research institutions engaged in biochip R&D and manufacturing in this region. The Midwest region encompassing states like Illinois, Michigan and Ohio has emerged as the fastest growing regional market growing at an annual rate of over 8% during the forecast period. Establishment of new manufacturing facilities by global corporations is driving the growth in the Midwest region.
*Note:
1. Source: Coherent Market Insights, Public sources, Desk research
2. We have leveraged AI tools to mine information and compile it